
Some properties of cathode rays: They travel in a straight path. While the historical persons and dates behind these experiments can be quite interesting, it is most important to understand the concepts resulting from their work. Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to create the image in a classic television set. The experimental results indicate that sulfur adsorption on LSC surfaces is omnipresent in the investigated conditions and even trace amounts of sulfur compounds present in nominally pure measurement gases account for particle formation and multiple degradation effects under operating conditions. Here, we will discuss some of those key developments, with an emphasis on application of the scientific method, as well as understanding how the experimental evidence was analyzed. Much of this came from the results of several seminal experiments that revealed the details of the internal structure of atoms. Link to Learning View this simulation of the Rutherford gold foil experiment. A small, relatively heavy, positively charged body, the nucleus, must be at the center of each atom.
#Summary and results of cathode ray experiment series#
In the two centuries since Dalton developed his ideas, scientists have made significant progress in furthering our understanding of atomic theory. Cathode Ray Experiment After Daltons breakthrough of atomic theory, scientists tried to determine the masses of atoms from the fraction of elements in compounds. Analyzing a series of such experiments in detail, Rutherford drew two conclusions: The volume occupied by an atom must consist of a large amount of empty space. And if atoms are neither created nor destroyed during a chemical change, then the total mass of matter present when matter changes from one type to another will remain constant (the law of conservation of matter). For example, if an element such as copper consists of only one kind of atom, then it cannot be broken down into simpler substances, that is, into substances composed of fewer types of atoms. Both the cathode and anode are positively charged. Both the cathode and anode are negatively charged.

The cathode is positively charged, and the anode is negatively charged. The cathode is negatively charged, and the anode is positively charged. I also said that one might claim that Thomson was the first to. The cathode and anode in a cathode ray tube carry which of the following charges a. The tube is evacuated to avoid electrons interacting with any particle. He came to know them during that year as a result of his experiments with cathode rays. (credit copper: modification of work by ).ĭalton’s atomic theory provides a microscopic explanation of the many macroscopic properties of matter that you’ve learned about. The thermionic emission is the process whereby metal surfaces emit electrons when heated. This was the beginning of further understanding leading to the atomic theory and structure that we know today.Ĭathode ray tube is a tube that contains a small amount of gasīetween two metallic plates.\): When the elements copper (a shiny, red-brown solid, shown here as brown spheres) and oxygen (a clear and colorless gas, shown here as red spheres) react, their atoms rearrange to form a compound containing copper and oxygen (a powdery, black solid). Thomson’s first experiment was to investigate whether or not the negative charge could be separated from the cathode rays by magnetism. He won the 1906 Nobel prize for the discovery of electrons.
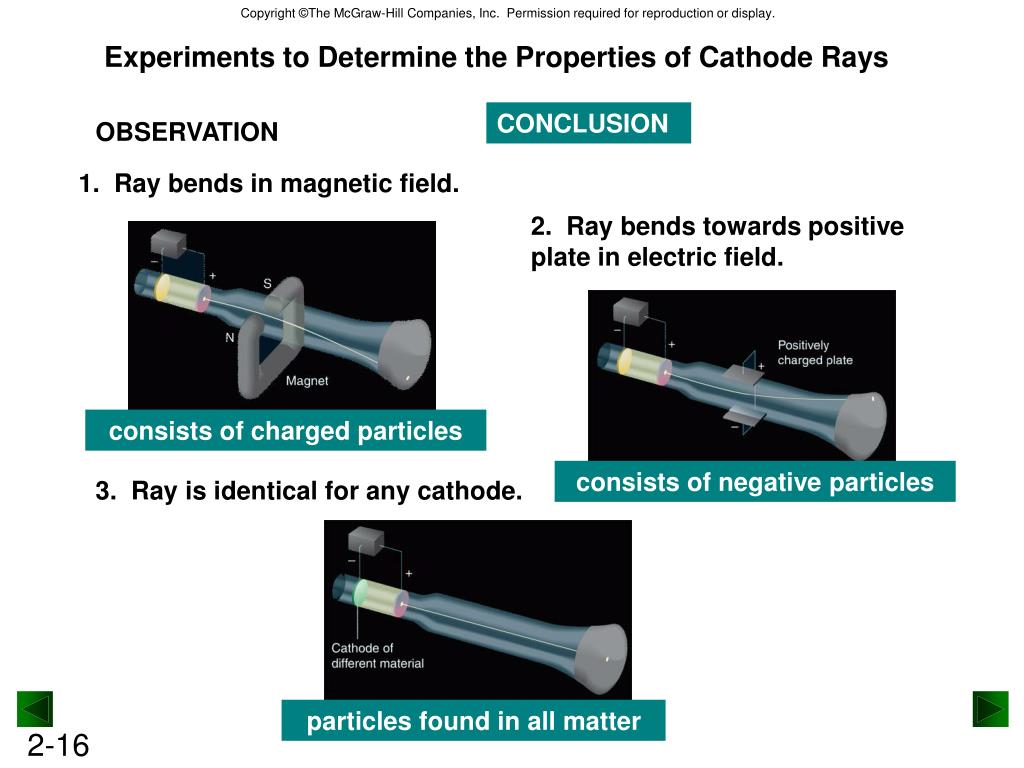
He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated.

Thomson, a British physicist, conducted the cathode ray experiment. CEI growth on epitaxial TMO thin film cathode Complete X-ray spectroscopy experiments of CEI growth on epitaxial TMO thin film cathodes Complete combined X-ray and MD analysis of electrolyte-model oxide interface Complete On track Complete On track Compare and co -verify in situ, ex situ X ray. Previously, atoms were known to be indivisible, but in 1897, J.


 0 kommentar(er)
0 kommentar(er)
